Technology
Nexavant®, the novel TLR3 agonist
-
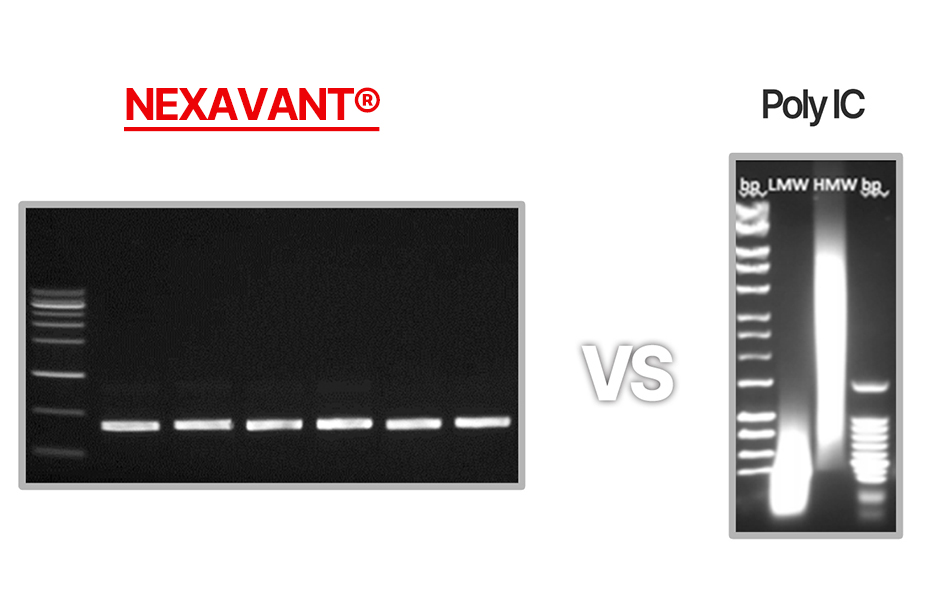
1 A defined TLR3 agonist with homogeneity
-
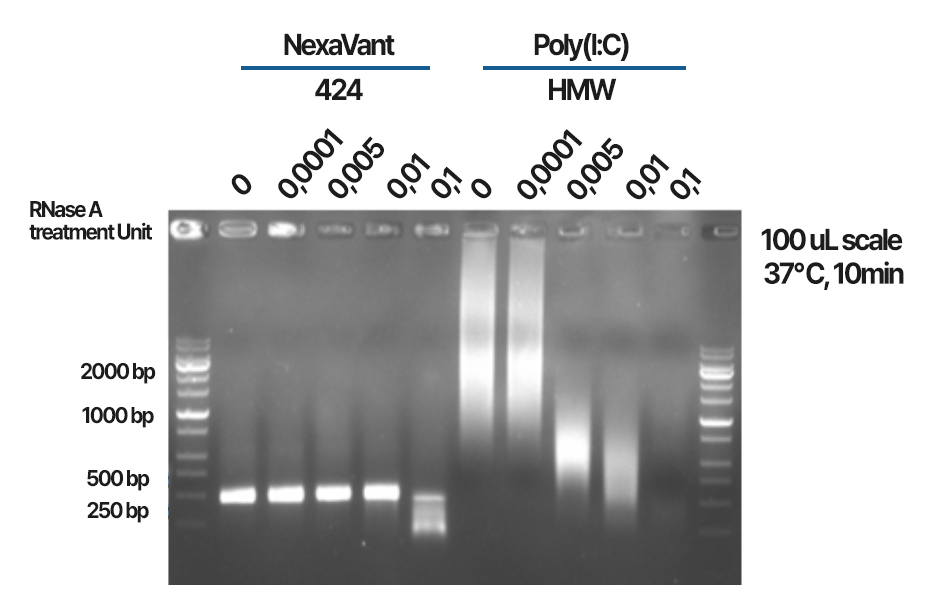
2 RNase A and heat resistant
-
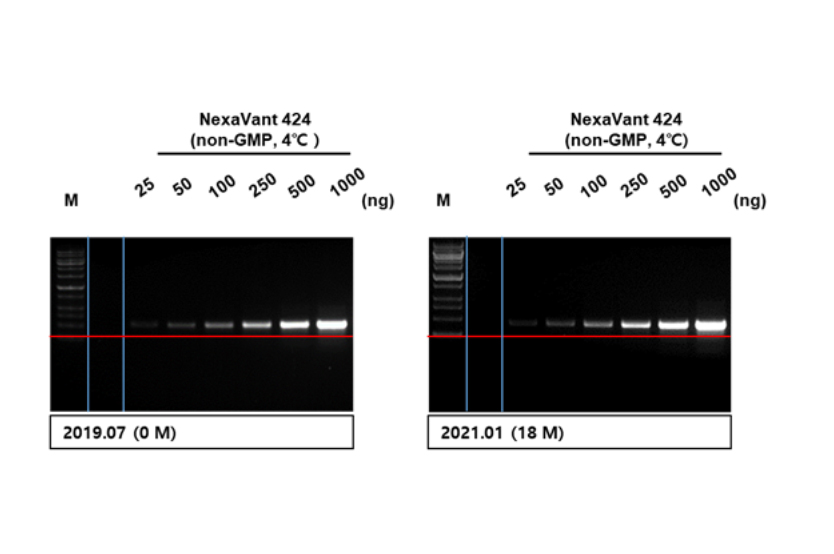
3Stable for more than two years at 42℃
-
4 TLR3 and RIGI agonist without MDA5 activation
-
5 Scale-up production in GMP grade
-
6 CAS registered (CAS Number: 2839526-76-8)
-
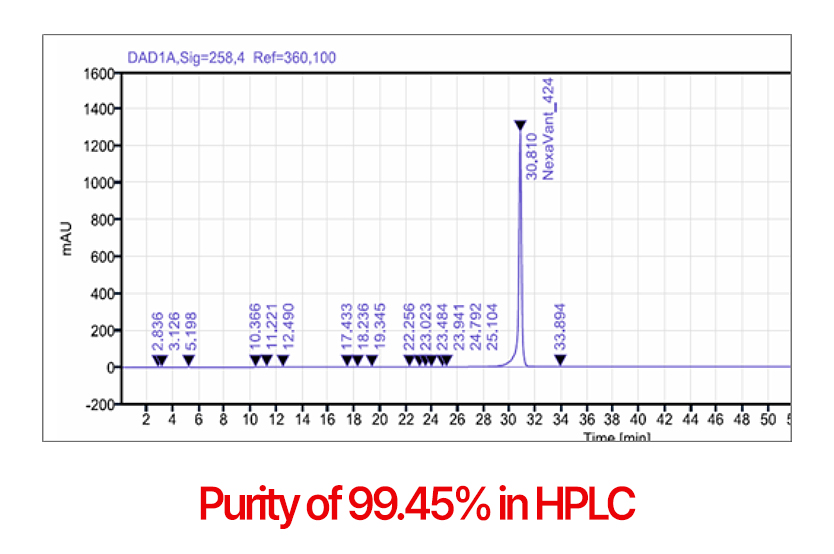
7 More than 99% purity in HPLC
-
8 Capable for more than 10 million dose production/year
-
9 Strong immune stimulant: https://pubmed.ncbi.nlm.nih.gov/36761735/
-
10 Potent anticancer effect: https://pubmed.ncbi.nlm.nih.gov/38136298/
-
11 Induce Th1 and CTL response
-
12 DS for the following development:
-
Vaccine adjuvant
-
Anti PD-1 dual therapy
-
Adjuvant for a cross-reactive flu vaccine
-
Peptide complex drug development

Nexavant®, a homologous, stable, and high purity product
-
Homogeneity
-
Stability I
-
Purity
-
Stability II